
Crosslinked Hyaluronic Acid Powder for Eye Drops
CrossYal is the first cross-linked hyaluronic acid for ophthalmic use, in powder form, offering heat stability and resistance to degradation.
Ophthalmic drops based on hyaluronic acid lose viscosity over time and generally reach the patient with reduced action and effectiveness. This is because ophthalmic drops use low concentration hyaluronic acid, which tends to degrade very easily, reducing viscosity and retention time on the corneal surface. Hyaluronic acid is also extremely sensitive to heat.
The technology used for CrossYal produces a cross-linked hyaluronic acid that is non-gelled and water soluble, in pure form, so that it can be used in the ophthalmic and pharmaceutical industries. Furthermore, the product obtained can be sterilized by0.2-micron filtration, and is therefore suitable for the industrial processes in the production of ophthalmic solutions.
Advantages of Crosslinked Hyaluronic Acid
Stability
Crosslinked hyaluronic acid is much more stable than linear hyaluronic acid. In the accelerated stability study, (test required to determine the rate of product degradation on the industrial scale), non-cross-linked linear hyaluronic acid suffered a loss of viscosity after the first 30 daysat 40 ° C ranging between 60 – 80%. After 90 days of accelerated stability at 40 ° C, the formulation containing CrossYal at a concentration of 0.15%, the cross-linked hyaluronic acid developed by Bioitech, registered a loss of viscosity of 10-12%. Other values, such as osmolarity and pH values, were also stable.
Accelerated stability test at 40°C
Physical degradation
Steam sterilization test at 121°C for 15 min
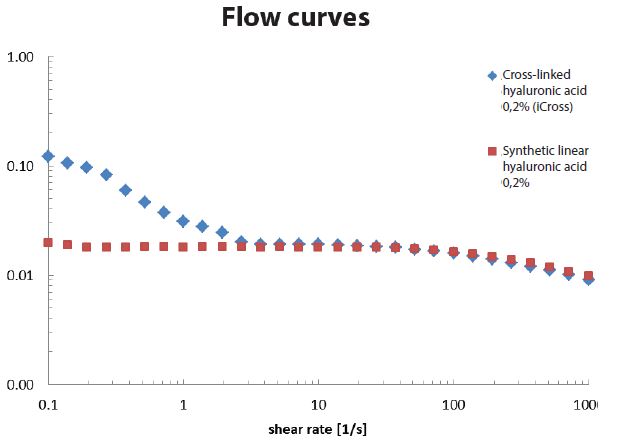
Rheological properties
This material published courtesy of: OFF Italia Srl – Oftalmica Farmaceutica Italia
Flow curves of 0.2% cross-linked hyaluronic acid vs 0.2% linear hyaluronic acid. At low shear rates, the crosslinked sample has a viscosity level that is 10 times higher. The substances behaved similarly at high shear rates.
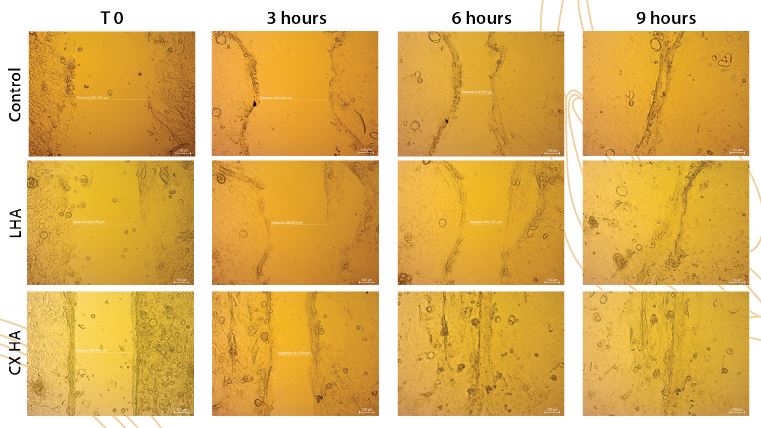
In Vitro proliferation and migration effects of corneal epithelial cells
Taken from: ADVANTAGES OF A CROSS-LINKED HYALURONIC ACID-BASED ARTIFICAL TEAR. Elbadawy HM, Ruzza A, Salvalaio G, Ferrari S, Fasolo A, Ponzin D. XIV SICSSO CONGRESS The International Congress of the Italian society Stem Cells and Ocular Surface. 25-27 June 2015 Lecce, Italy
Cells treated with Crosslinked Hyaluronic Acid (CX HA) show faster regrowth and this can be an advantage in cases of corneal ulcers
Why CrossYal is more effective than linear hyaluronic acids for ophthalmic use
The technology studied for CrossYal is based on:
- molecular weight optimization with 85% of the chains above 1 million daltons and average weight of about 2.5 million daltons in the presence of crosslinking, which guarantees industrial filtration
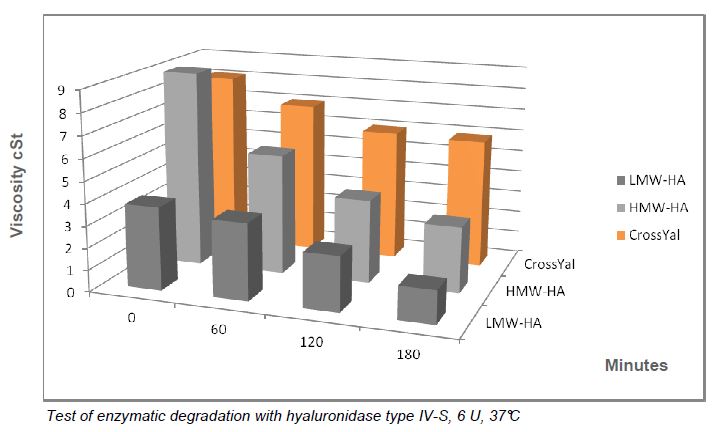
- change in the allosteric binding configuration of specific enzymes (hyaluronidase) caused by crosslinking and slowdown of degradation process, enhancing the product’s final efficacy
Scientific Publications
Synonym: Crosslinked Sodium Hyaluronate | Linear Formula: (C14H21NO11)n | Molecolar Weight: 2,5 MDa
Safety Data Sheet of Raw Materials MSDS

